Canine Parvovirus(CPV) antigen rapid test card
1. Product Usage
Canine parvovirus (CPV) disease is a potent canine infection that is highly contagious. The pathogen is canine parvovirus, mainly CPV-2. The virus mainly infects dogs, especially puppies, which are highly contagious, have an acute onset, and have a high mortality rate. The disease is usually caused by intestinal infection, with fecal-oral infection as the main route of transmission, and susceptible animals are mainly infected by direct or indirect contact. This product is tested by immunochromatography on canine feces or vomit samples to quickly detect whether a dog has been infected with canine parvovirus.
2. Principle
This product uses rapid immunochromatography to detect CPV antigen. After the diluted sample is added to the “S” hole on test card, if the CPV antigen is present in the sample, the antigen will specifically bind to the colloidal gold-labeled CPV monoclonal antibody, and the complex will move along the chromatographic membrane and be chromatographed. The pre-coated CPV monoclonal antibody on the membrane is captured, and a wine red detection line is formed at the T-position of the cartridge. If there is no CPV antigen in the sample, no visible lines will be formed at the T line. In addition, the system has also designed a C-line for verifying that the test is valid—whether negative or positive, the line should be colored, otherwise it will be invalid.
3. Components
1)CPV Ag Test Card ( Including 1 CPV Ag test cassette, 1 dropper, 1 desiccant) 10 pieces
2) Sample buffer (1ml/tube) 10 tubes
3) Swab 10 pieces
4) Instruction 1 piece
4. Test Procedure
1) Read the manual carefully and return the test card, sample into room temperature 15~25℃before use.
2) Sample collection: Use a cotton swab to collect a sample of freshly excreted feces or vomit, or take a sample directly from the rectum. Immediately insert the cotton swab with the secretion into the sample tube containing the sample buffer and shake vigorously. Stand for a few minutes and ready to test. (Note: When the sampling amount is too much, it will affect the stability of the colloidal gold particles, which may cause false positive. The sample size should cover 1/3 to 2/3 of the cotton swab.)
3) Open the package, take out test card, put it on desk flatly.
4) Use the supplied dropper to absorb the diluted sample, drop 3~4 drops (about 100ul) into “S” hole on test card.
5) Read the result in 5~10min, result is invalid beyond 15 min.
5. Result judgment
1) Positive(+): Both on T Line and C line being seen wine-red color reaction, it means there is CPV antigen in the sample.
2) Negative(-): no color reaction on Test Line (T Line),only on Control Line(C Line) being seen wine-red color reaction, it means there is no CPV antigen in the sample.
3) Invalid(X): No color reaction on C Line, it means the operation is wrong or the test card is invalid, test again.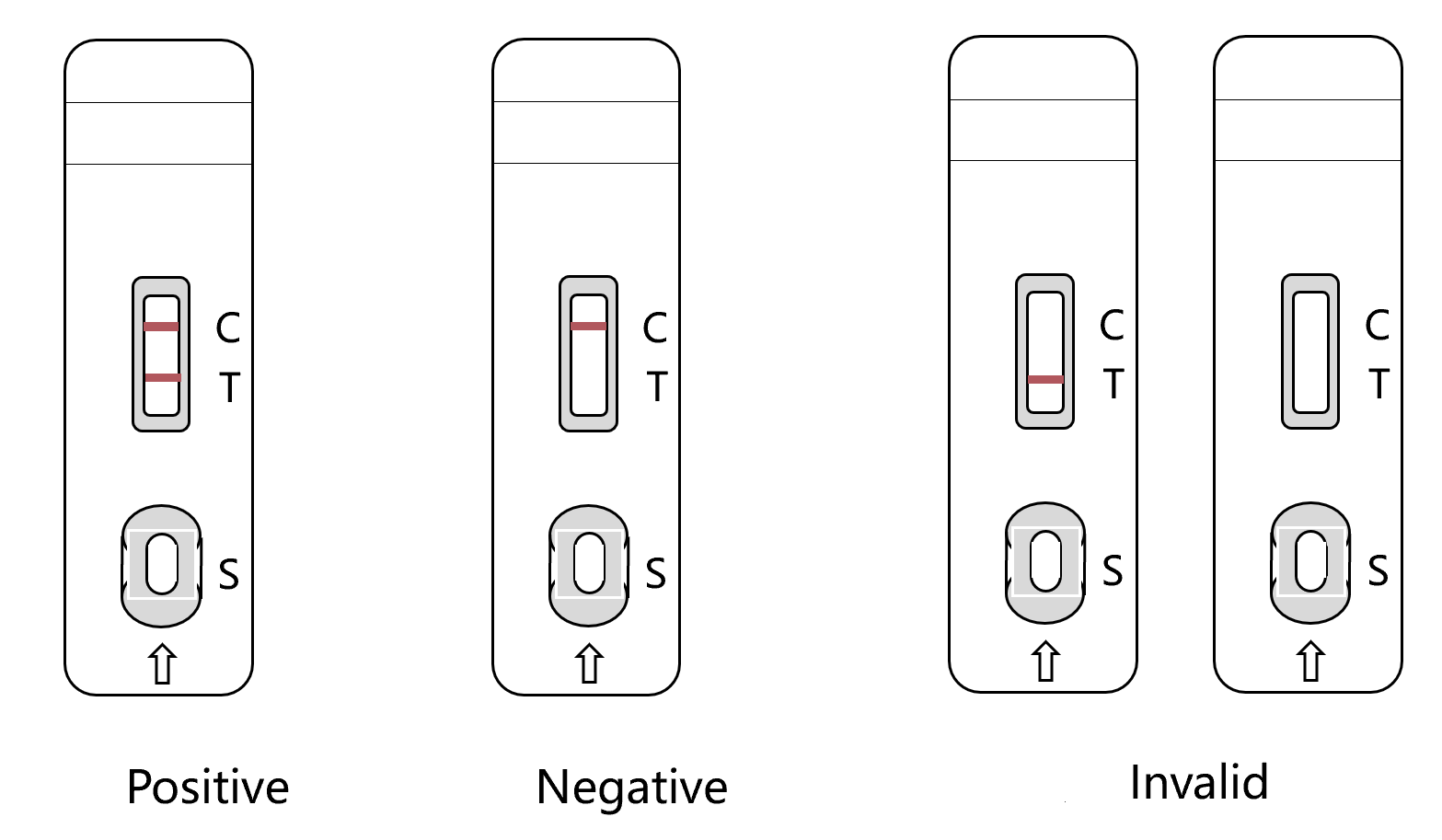
6. Specifications, Storage and Stability.
Package: 10T/box
Store at 4~30℃ in a cool, dark and dry place,no frozen. Shelf life: 18 months.
7. Precautions
1) Please use the test card within the shelf life and within 1 hour after opening.
2) Avoid direct sunlight and direct fan blowing during testing.
3) Try not to touch the white film surface of the non-plastic part of the test card.
4) Sample droppers should not be mixed to avoid cross contamination.
5) Do not use sample buffer that are not supplied with this reagent.
6) When the detected substance content is very high, the T line may appear, and then the red color will gradually fade or even disappear. It is recommended to dilute the sample by 10 times before testing, so that the T line can be stable.
7)The used test card should be treated as microbiological dangerous goods.
8.Application limitations
This product is an immunological diagnostic kit, which is only used to provide qualitative test results for clinical testing of pet diseases. If there is any doubt about the test results, please use other methods of diagnosis (such as RT-PCR, Western-blot, etc.) to further analyze the samples. For pathological analysis, please consult your local pet physician.

